


 U.Mars — Encyclopedia
U.Mars — Encyclopedia
Basic Astronomy and the Nighttime Sky
There are three main types of spectra: continuum, emission, and absorption.
Recall blackbody radiation, wherein an object emits radiation over a broad range of wavelengths, depending on its effective temperature; e.g., everyday room-temperature objects, and animals and humans, emit mostly over the infrared region (and longer wavelengths), while hot objects like incandescent light bulb filaments and stars emit brightly in visible light.
A continuum spectrum is produced by such objects, recording broad-spectrum emission; the objects' peak emission at around a certain color may manifest as a brighter image in a particular region of the spectrum, but overall there will be a smooth transition between colors.
example continuum spectrum, for an object at about ˜ 3,000 K |
 |
for an object at about ˜ 6,000 K |
 |
for an object at about ˜ 10,000 K |
 |
Emission spectra come from the specific energies of electron level transitions in the atoms of an object. The electrons drop from an excited energy level to a lower one, releasing a photon corresponding to the energy difference. That photon's energy may be detected as a thin bright point in an otherwise-dark spectrum. Several electron-level transitions going on together in a group of atoms of a certain chemical element (e.g., an excited cloud of hydrogen gas, in a star-forming region) will yield an emission spectrum with several bright lines at wavelengths specific to those transitions. In this way an emission spectrum is a "fingerprint" to the existence of a certain element in a sample.
example emission spectra, for Hydrogen gas |
 |
for Helium |
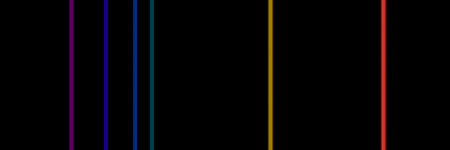 |
These are the result of detecting a continuum source on the other side of a cloud of unexcited gas. The intervening material preferentially absorbs photons of energies corresponding to its electron energy level transitions, effectively removing those colors from the continuum spectrum seen on the other side of the source; the excited gas does re-emit the photons, eventually, though not in the same direction as those photons were originally traveling.
Stars have cooler hydrogen and helium, and trace other other elements, in their outer atmospheres above the hotter regions responsible for most of their visible light output. As a result, stars have very intricate absorption spectra.
example absorption spectra, counterpart to the emission spectra above, for Hydrogen gas in front of a ˜ 6000 K blackbody emitter |
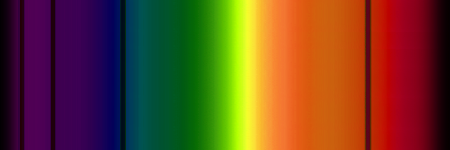 |
for Helium |
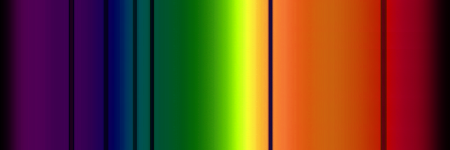 |
See also: